Table of Contents
OBJECTIVES
After attending to this experiment, we shall be able to learn moisture content determination in food products using karl- fischer titration method.
INTRODUCTION
The Karl Fischer Titration method is particularly suitable for food in which heating methods give erratic results. This method has been approved for dried vegetables, oils and fats, cocoa products and liquid molasses. This method determines the total moisture (bound +free) content. It is suitable for food products containing 2% or less of moisture content.
PRINCIPLE
The determination of water is based on the reaction of water while oxidizing R-sulphite anion to R-sulphate by iodine.
The reaction of sulphur dioxide with alcohol (methanol) producing a mono-alkyl ester of the sulphurous acid is a basic requirement for the Karl Fischer reaction.
The mono-alkyl ester of sulphurous acid in turn reacts with water in presence of iodine and amine to form stable salts as per the following equations :
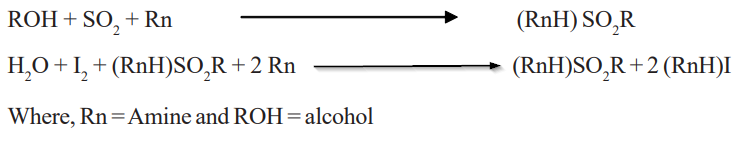
REQUIREMENTS
Reagents
Methanol/2 – Methoxyethanol
Sample Solvent : Either methanol or a mixture containing 4 parts of methanol and 1 part of pyridine (by volume) or (preferably for determination with compounds containing carbonyl groups) a mixture containing 4 parts of 2-methoxyethanol and 1 part of pyridine (by volume).
In special cases, other solvents may be recommended, e.g., acetic acid, pyridine or a mixture containing 1 part of methanol and 3 parts of chloroform (by volume)
Karl Fischer Reagent – Sodium Tartarate, Crystalline shall be of such quality that when dried at 150°C for 3 hours, it gives out 15.66 + 0.5% water.
APPARATUS
Any commercial Karl Fischer titrator with electrometric end point detection will be suitable. The instrument should incorporate the following:
Automatic Burette – 10 to 25 ml capacity with a fine pointed tip and graduations of 0.05 ml.
Titration Vessel – 100 ml capacity having provision for inserting burette platinum electrodes and also for the introduction of liquid samples (suitable ground glass joint stopper) with the help of syringe or pipette, without removing the vessel from apparatus.
A similar arrangement for introduction of solid samples with least exposure is preferred.
Reagent bottle for Karl Fischer Reagent – Amber coloured bottle connected to automatic burette through ground glass joint.
Double platinum electrode.
Magnetic stirrer with PTFE coated stirring bar.
Electrometric end point detection device.
Glass syringe -suitable capacity.
A small glass tube – closed at one end and fitted at the other with a rubber stopper, used for weighing and introducing into the titration vessel. For example, the mass of crystalline sodium tartarate approximately 200 mg) used to standardize the Karl Fischer reagent or possibly test samples of solid products.
PROCEDURE
Standardization of Karl Fischer Reagent
Add 1 drop of water. Titrate the known quantity of water thus introduced with the Karl Fischer reagent until the end point is reached. Note the volume of reagent used.
Water equivalent (T) of the Karl Fischer reagent (mg H2O/ml) = (M/A)
Where,
M = mass, in mg, of water used, or mass, in mg, of Na tartarate introduced multiplied by 0.1566, and
A = volume, in ml, of Karl Fischer reagent used.
Titration with Sample
Empty the titration vessel by mean of the emptying tap. Place in it 25 ml of methanol or other solvent suitable for the products to be analysed, using a syringe passing through the ground glass stopper. Switch on the electromagnetic stirrer.
Start adding Karl Fischer reagent, until the end point is reached. Then introduce the required amount of test portion sample by means of a syringe in the case of a liquid or weighed to the nearest 0.1 mg in a small weighing bottle in the case of a solid powder.
Titrate with Karl Fischer reagent using the same electrometric procedure for detecting the end point of the reaction. Note the volume of Karl Fischer reagent for the determination.
CALCULATION
Water, % by mass= (B × T)/(M × 10) or, (B × T)/(V × D × 10)
Where,
B = volume, in ml, of Karl Fischer reagent used for the test,
T = water equivalent, in mg/ml, of the Karl Fischer reagent,
M = mass, in g, of the test portion (for solid products),
V = volume, in ml, of the test portion (for liquid products), and
D = density of the sample, in g/ml, at measurement temperature (for liquid products
only.
RESULTS AND INFERENCE
The difference between results of two concurrent determinations carried out simultaneously or in rapid succession by the same analyst (repeatability) shall be ±0.1% by mass.
PRECAUTIONS
• Handle Karl Fishcher reagent with care.
• Proper ventilation should be there while doing the experiment.
• Use only the appropriate solvent for sample dissolution.
• Clean the Karl Fischer Titrator properly before and after use.
