Table of Contents
INTRODUCTION
The Acid value has wide implications in the oil refining industry.
It conveys not only the quality of oil but also the total quantity of alkali needed to neutralize the acidity in a particular batch for making it suitable for the purpose of hydrogenation or marketing of refined oil or fat of very low acidity.
The acid value is a measure of the hydrolytic rancidity present in the sample.
PRINCIPLE
- The acid value is determined by directly titrating the material in an alcoholic medium with aqueous sodium or potassium hydroxide solution.
- The acid value is the number of mg of KOH required to neutralize the free fatty acids present in 1 g of the oil or fat.
- The free fatty acid is calculated as oleic, lauric, ricinoleic, or palmitic acids.
REQUIREMENTS
Reagents Ethyl Alcohol – 95%v/v, neutral to phenolphthalein indicator.
- Phenolphthalein Indicator Solution – Dissolve 1 g of phenolphthalein in 100 ml of ethyl alcohol.
- Note: When testing oils or fats which give dark-colored soap solution, the observation of the endpoint of the titration may be facilitated either
(a) by using thymolphthalein or alkali blue 6B in place of phenolphthalein, or
(b) by adding 1 ml of a 0.1%, w/v solution of methylene blue in water to every 100 ml of phenolphthalein indicator solution before the titration.
- Standard Aqueous Potassium Hydroxide or Sodium Hydroxide Solutions – 0.1 N or 0.5 N
PROCEDURE
- Mix the oil or melted fat thoroughly before weighing. Weigh accurately a suitable quantity of the cooled oil or fat in a 200 ml conical flask.
- The weight of the oil or fat taken for the test and the strength of the alkali used for the titration shall be such that the volume of alkali required for the titration does not exceed 10 ml.
- Add 50 to 100 ml of freshly neutralized hot ethyl alcohol, and about 1 ml of phenolphthalein indicator solution.
- Boil the mixture for about five minutes and titrate while as hot as possible with standard aqueous alkali solution, shaking vigorously during titration.
CALCULATION

Where,
V = volume in ml of standard KOH/NaOH solution used,
N = normality of standard KOH/NaOH solution, and
W = weight in g of the material taken for the test.
RESULTS AND INFERENCE
The difference between the results of two determinations carried out simultaneously or in rapid succession by the same analyst (repeatability) shall not exceed 0.1.
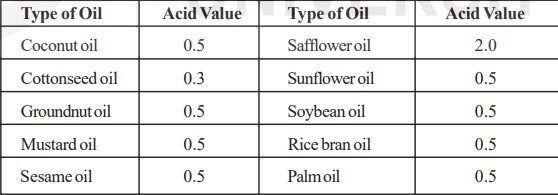
The acid value of commonly used edible oils is given as below.
PRECAUTIONS
• The formation of two layers should be avoided by vigorous shaking so that the free acids do not get transferred into the ethanolic layer.
• The freshly neutralized alcohol must also be hot at the time of addition.
• The weight of the oil or fat taken for acidity determination and the strength of NaOH should be such that the volume of alkali used does not exceed 10 ml.
