Table of Contents
INTRODUCTION
The starch by enzymatic method is a measurement of digestible starch.
Digestible starch has a great significant role, since it results in the production of alcohol, sugar, maltodextrin, etc.
Digestibility of starch is directly linked to glycemic index of starch.
PRINCIPLE
Test portions are hydrated and starch is gelatinized followed by hydrolysis into glucose with glucoamylase at 55°C. Glucose is determined with glucose oxidase-peroxidase reagent.
Reagents
- Glucoamylase solution – 10mg (30 IU/ml) in water
- D-Glucose standard solution – Dissolve 400 mg anahydrous glucose in 1 litre of distilled water and let stand for 4 h to complete mutarotation before use.
- Acetate buffer (4M, pH 4.8) – Dilute 120 ml glacial acetic acid ( CH3 COOH) and 164 g anhydrous sodium acetate (CH3 COONa) to 1 liter with distilled water.
- Tris-Phosphate buffer – (pH 7.0) – Dissolve 36.3 g trihydroxy methyl aminomethane and 50 g NaH2 PO4 .H2O in 500 ml water. Adjust pH to 7 with H3 PO4 at 370 C and dilute to 1 litre with water.
- Enzyme-buffer chromogen mixture – Dissolve 30 mg glucose-oxidase, 3 mg peroxidase and 10 mg O-dianisidine. 2HCl in 100 ml tris-phosphate buffer. Disperse O-dianisidine.2HCl completely in small amount of buffer before adding it to enzyme – buffer mixture.
PROCEDURE
- Grind test sample to less than 0.5 mm size by passing through sieve.
- Determine moisture content of the sample.
- Weigh 0.5 g sample in 250 ml dried and weighed conical flask.
- Add 25 ml of water with stirring and adjust pH to 5.7. Boil gently for 3 min. and then autoclave for 1 hr at 1210 C.
- Remove from autoclave, cool to 550 C. Add 2.5 ml acetate buffer and sufficient water to total weight of solution of 45±1 g.
- Immerse flask in water bath with shaker at 550 C± 10 C and add 5 ml glucoamylase solution.
- Hydrolyze for 2 hr with continuous shaking, filter through filter paper into 250ml volumetric flask, wash quantitatively, dilute to volume.
- Transfer 1ml aliquot (containing 20-60 μg D glucose) to test tube.
- Add 2 ml enzyme buffer chromogen mixture. Shake tubes and place in dark at 37±10 C for 30 min. to develop colour.
- Stop reaction by adding 2 ml H2 SO4 (1:1) and measure absorbance at 540 nm.
- Prepare standard curve from 0-60 μg D glucose/ml and blank
CALCULATION
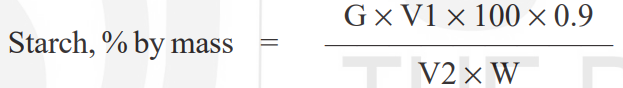
Where,
G = glucose content (μg) calculated from standard curve,
0.9 = Conversion factor of glucose into starch,
V1 = aliquot of sample solution (ml) taken for colour development,
V2 = final volume (ml) made after hydrolysis of starch, and
W = weight, in g, of test sample.
RESULTS AND INFERENCE
The difference between the results of two concurrent determinations carried out simultaneously or in rapid succession by the same analyst (repeatability) shall not exceed 0.5 % by mass.
PRECAUTIONS
• Enzyme should be free from glucose.
• Experimental conditions like temperature, pH, incubation period mentioned in
standard procedure should be followed strictly.
• Check the activity of enzymes before analysis of sample.
