Table of Contents
OBJECTIVES
To determine protein content in food products by Kjeldahl method
INTRODUCTION
The Kjeldahl method has a wide acceptance for the determination of protein in food products.
The protein content of foods is usually calculated from total nitrogen by multiplying with a suitable conversion factor that is based upon the nitrogen % present in a particular protein
PRINCIPLE
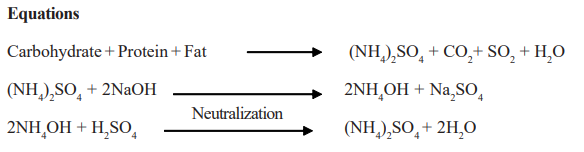
The sample is oxidized in the presence of sulphuric acid and nitrogenous compounds are converted into ammonium sulphate.
Mercury is added to the digestion mixture as a catalyst and alkali sulphate as a boiling point elevator.
Ammonia is liberated by adding an excess of alkali and is quantitatively distilled into a measured volume of standard hydrochloric or sulphuric acid.
The acid not neutralized by ammonia is back-titrated with standard alkali to give a measure of the nitrogen content in the sample.
REQUIREMENTS
Apparatus
For Digestion
(A) Kjeldahl flasks (500 to 800 ml capacity)
(B) A heating device (heater/burner)
For Distillation
A) Round bottom flask (1 litre capacity)
B) Splash head
C) Condenser (Allihn type)
D) Trap
E) Beaker (500 ml capacity)
F) Receiving funnel
Reagents
-Concentrated Sulphuric Acid, AR Grade
-Potassium Sulphate or Anhydrous Sodium Sulphate, AR Grade
-Sodium Hydroxide Solution – Dissolve about 450 g solid sodium hydroxide in distilled water, cool, and dilute for 1 litre. The specific gravity should be at least 1.36 at 20°C.
-Hydrochloric or Sulphuric Acid, Standard Solution: (0.1N or 0.5N).
-Prepare the standard solution as discussed in Experiment No.1 (part-B). Standardize against sodium hydroxide standard solution.
-Sodium Hydroxide Standard Solution – 0.1 N. Standardize against primary standard and against standard acid solution.
-Methyl Red Indicator – Dissolve 1 g methyl red in 200 ml alcohol.
PROCEDURE
Digestion
Accurately weigh 0.7 to 2.2 g of the sample into the digestion flask. Add 0.7g mercury oxide or 0.65 g mercury and 15 g powdered potassium sulphate or anhydrous sodium sulphate, and 25 ml sulphuric acid.
Ratio of salt to acid (m/v) should be approximately 1: 1 at the end of digestion for proper temperature control.
Digestion may be incomplete at a lower ratio and nitrogen may be lost at a higher ratio.
Each gram of fat consumes 10 ml and each gram of carbohydrate consumes 4 ml sulphuric acid during digestion.
Place the flask in an inclined position on a heater and heat gently until foaming ceases.
A small amount of paraffin or silicon antifoam may be added to reduce foaming. Boil vigorously until the solution becomes clear and then continue boiling it for 1 to 2 hours.
Distillation
Cool, add about 200 ml distilled water, and in order to avoid complex formation, add 25 ml of the sulphide or thiosulphate solution.
Mix to precipitate the mercury.
Add a few zinc granules to prevent bumping, incline flask, and add without agitation 25 g of sodium hydroxide as solid or equivalent as solution, to make solution strongly alkaline (thiosulphate or sulphide solution may be mixed with the sodium hydroxide solution before addition to the flask).
Immediately connect flask to distillation bulb or trap on condenser, and with tip of the condenser immersed in a measured quantity standard acid (usually 50 ml, 0.5 N or an appropriate quantity of 0.1 N ) in the receiver, rotate flask to mix the contents thoroughly; then heat immediately until all ammonia has distilled over (at least 150 ml distillate).
Lower the receiver before stopping distillation and wash tip of condenser with distilled water.
Back titrate excess acid with standard 0.1 N sodium hydroxide, using methyl red as indicator. Correct for blank determination in reagents.
Blank – Conduct determinations using all reagents and 2 g of sugar.
CALCULATION
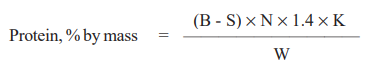
where,
B = volume, in ml, 0.1 N alkali used for titration for blank,
S = volume, in ml 0.1 N alkali used for titration for sample
N = normality of alkali used for titration,
K = Kjeldahl factor, and
W = weight, in g, of sample taken for test.
RESULTS AND INFERENCE
Duplicate determinations of the nitrogen should agree within 0.05% nitrogen. Appropriate conversion factor for protein from nitrogen should be used for specific food samples.
The following Kjeldahl factors are used for different food products in converting nitrogen to protein.
| Sno | Types of Food Material | Kjeldahl Factor |
| 1 | Rye, oat meal, whole wheat | 5.83 |
| 2 | Wheat flour and its products viz. bread, macaroni, spaghetti | 5.70 |
| 3 | Maize, rice polish, pulses, tea, cocoa, coffee, malt, beer, etc. | 6.25 |
| 4 | Groundnut, brazil nut | 5.46 |
| 5 | Cashew, coconut and other tree nuts, sesame, safflower, sunflower, castor, cottonseed, linseed | 5.30 |
| 6 | Milk and milk products, margarine | 6.38 |
| 7 | Egg whole, egg powder | 6.68 |
PRECAUTIONS
• Sample to be analyzed should be homogeneous.
• Determine the strength of NaOH before use.
• Sample should be checked for complete digestion through colour and there should not be any presence of carbon particles adhering to the neck of Kjeldahl flask.

