Table of Contents
INTRODUCTION
The titer of an oil or fat is the solidifying point of the mixed component fatty acids.
The titer temperature is a means for characterizing oils and fats and for assessing the hardness.
The titer test is related to the degree of unsaturation of the fatty acids and increases with increasing amounts of unsaturated fatty acids present.
PRINCIPLE
The fatty oil is saponified with glycerol-caustic potash solution, and the soap is acidified with dilute sulphuric acid to give fatty acids.
The fatty acids obtained are washed and dried. The highest temperature recorded during the solidification under standard conditions is the titer.
REQUIREMENTS
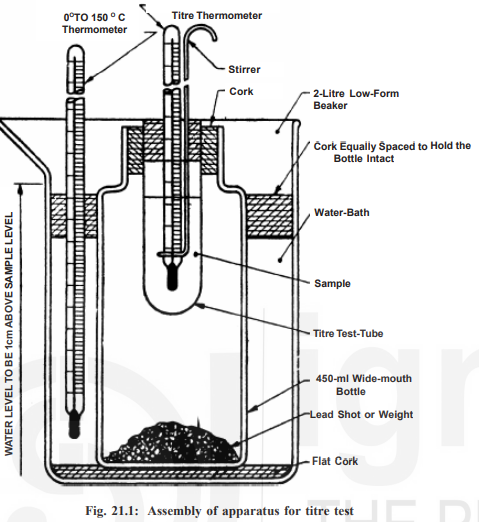
Apparatus
Saponification Flask – 250 to 300 ml capacity.
Low form beaker – (2 liter capacity) to serve as a water bath.
Wide-Mouth Bottle – 450 ml capacity (190 mm height and inner dia of neck 38 mm).
Titre test tube – (length 100 mm, dia. 25 mm, and 1 mm thickness.
Stirrer – (glass or stainless steel), with one end bent in the form of a loop of 19 mm outer diameter. The upper end may be formed to suit stirring with a hand or attached to a mechanical stirrer.
Laboratory Thermometer – range 0 to 150°C.
Titre Thermometer – (−2° to 68°C with LC 0.2°C).
- Reagents
- Glycerol-Caustic Potash Solution – Dissolve 250 g of KOH in 1250 g of glycerine with the aid of heat. Do not heat >140°C.
- Dilute Sulphuric Acid – 30 % by mass obtained by cautiously adding 16 ml of conc. H2
- SO4 (sp gr 1.84) to 70 ml of water.
- Methyl Orange Indicator Solution – Dissolve 0.1 g of methyl orange in 100 ml of water.
- Acetone
PROCEDURE
Preparation of Fatty Acids:
- Weigh about 70 g of the glycerol caustic potash solution into the saponification flask.
- Heat to 150°C while stirring. Add about 30 g of the sample of oil or melted-fat and re-heat to about 150°C.
- If necessary, add a little more glycerol-caustic potash solution to ensure complete saponification. (Complete saponification is usually indicated by an initial change in the appearance of the mass often accompanied by an increase in the viscosity or thickening.
- The solution then thins out after the reaction is complete and assumes a homogeneous appearance.
- The most common characteristic is that of soap bubbles forming and rising from the surface. Considerable care should be exercised at all times to ensure complete saponification.)
- Cool slightly and dissolve the soap in 300 ml of water contained in a 1000 ml beaker.
- Add dilute sulphuric acid until the solution is distinctly acidic to methyl orange indicator, and place the beaker in a boiling water bath until the fatty acids collect as a clear layer at the top.
- Siphon off the lower aqueous acid layer, add 300 ml of hot water, place in the boiling water bath for a few minutes, and again siphon off the aqueous acid layer.
- Wash the fatty acids thrice in this manner or until the last traces of soap and acid are removed. The acidification and washing should be done in as short a period as possible, keeping the beaker covered to prevent the oxidation of the fatty acids.
- After the last wash, allow the fatty acids to settle for a few minutes and then decant them carefully.
- Filter through one or two thicknesses of filter paper introduced into a conical flask, and add about 10 ml of acetone. Close the flask with an airtight cork, carrying a glass tube.
- Immerse the flask in boiling water and apply suction from a water pump until all bubbling ceases. Remove the cork and dry the contents of the flask at about 105°C for at least half an hour.
Determination of Titre
- Fill the low-form beaker with water up to two-thirds of its capacity.
- Adjust the temperature of water between 15°C and 20°C below the expected titer point when it is not above 35°C, and at 20 ± 1°C when it is 35°C or higher.
- Fill the test tube up to the mark with the fatty acid preparation at a temperature 10 to 12°C higher than the expected titer point.
- Insert the titer thermometer in the center of the sample and adjust its height so that its immersion mark coincides with the top surface of the fatty acid layer.
- When the temperature of the fatty acid comes down to about 10°C higher than the titer point, set the stirrer moves in a vertical direction at a rate of about 60 complete up and down motions per minute.
- The temperature of the fatty acid gradually comes down and stirring is continued until the temperature remains constant for 30 seconds.
- The stirring is stopped when the temperature begins to rise and the stirrer is raised out of the sample. The highest temperature recorded by the thermometer during this rise is the titer point.
RESULTS AND INFERENCE
The mean of the results of two determinations should be taken.
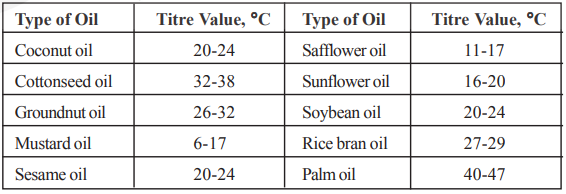
Report the data only when the agreement is reached on duplicate tests within 0.2°C. The titer value indicates the purity of the oil and is specific for a particular oil.
The refractive index of commonly used edible oils is given below.
PRECAUTIONS
• Care must be taken that saponification is complete in all cases. This is usually indicated by a change in the appearance of the soap, which finally becomes homogenous.
• In some cases, the further addition of a small quantity of caustic-glycerol may be necessary to obtain complete saponification.
