Table of Contents
INTRODUCTION
Acetyl value refers to the number of mg of KOH required to neutralize the acetic acid liberated by the hydrolysis of 1 g of acetylated oil or fat.
Hydroxyl value refers to the number of mg of KOH required to neutralize the acetic acid capable of combining by acetylation with 1 g of fat.
PRINCIPLE
- The process consists of acetylating the oil or fat with a measured quantity of acetic anhydride in pyridine decomposing the excess anhydride by boiling with water and then, after the addition of sufficient butyl alcohol to give a homogeneous solution, titrating with alcoholic alkali.
- A control test with the acetic anhydride and pyridine without the oil or fat provides a measure of the acetic anhydride available for acetylation; a similar test with the oil or fat and the pyridine without the acetic anhydride provides a measure of the free fatty acid present.
- From the figures obtained, the acetyl value or the hydroxyl value of the fat is calculated.
REQUIREMENTS
Reagents
Pyridine – Reflux with powdered barium oxide and distil. Use the fraction distilling above 114°C.
Acetic anhydride
Acetylating agent – Mix one volume of acetic anhydride and three volumes of pyridine.
Alcoholic sodium hydroxide solution – Prepare by dissolving sufficient aqueous NaOH (60 %, w/v) in 95 percent alcohol to make a 0.30 to 0.35 N solution. Remove the precipitated carbonate by filtering. The solution should be standardized against standard acid in the presence of phenolphthalein before use. The solution remains colorless fora long time if kept below 25°C.
n-Butyl alcohol
Phenolphthalein solution – Dissolve 0.1 g in 100 ml of 60% rectified spirit.
Note: In the determination of substances giving dark-colored soap solutions.
Observation of the endpoint of the titration may be facilitated either (a) by the substitution of thymolphthalein or alkali blue 6B for phenolphthalein or (b) by the addition of 1 ml of 0.1 percent solution of methylene blue to each 100 ml of the phenolphthalein solution before the titration.
Apparatus
A round-bottom flask 150 to 200 ml capacity with a 100 cm ground-in air condenser tube.
PROCEDURE
Weigh accurately 0.5 to 3.0 g of fat in the acetylation flask.
Measure, or preferably weigh, from a 10 ml burette into the flask, 5 ml of the pyridine-acetic anhydride mixture.
Add the mixture dropwise, and allow no time for drainage.
Before attaching the condenser, moisten the neck of the flask with pyridine to act as a seal, and make sure that the seal is maintained during the acetylation.
Mix the fat and the acetylating agent by shaking well.
Add one or two small pieces of pumice stone and heat the contents of the flask on a steam bath under a reflux condenser for 60 minutes.
Cool the flask to about 50°C and with a rotary motion to assist in washing the condenser tube, add 5 ml of distilled water from the top of the condenser.
Shake the mixture well, and then boil it gently for 5 to 10 minutes, shaking the flask two or three times during the boiling.
After cooling the flask and the contents to room temperature and before detaching the condenser, wash the condenser with 30 ml of butyl alcohol.
Detach the condenser and wash the neck and mouth of the flask and the tip of the condenser with a further 20 ml and then, if the contents of the flask are not homogeneous, add butyl alcohol until they become homogeneous.
Titrate the free acetic acid with carbonate-free 0.35 N NaOH solution, in the presence of a few drops of phenolphthalein as an indicator.
Carry out the same series of operations with 5 ml of pyridine acetic anhydride mixture alone, also with a corresponding weight of the oil or fat plus 5 ml of pyridine.
CALCULATION
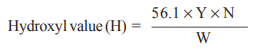
Where,
N = normality of NaOH solution;
Y = volume of NaOH solution in ml corresponding to the amount of acetylated fat formed = a + b – c (where, a, b and c are, respectively, the volumes in ml of NaOH required by blank with pyridine-acetic anhydride mixture, fat plus pyridine, and fat plus pyridine and acetic anhydride;
and W = weight in g of fat or oil taken for the test.
Acetyl value = (H/ 1+0.0075 H)
Note: It is to be noted that a slight increase in the acetyl value has been found to occur with increasing the free fatty acid content of the sample.
RESULTS AND INFERENCE
The mean of the results of two concurrent determinations should be reported.
Duplicate saponification values of the sample or acetylated product should not differ by more than 0.2.
The acetyl value of common fat and oils ranges from 2.5 to 20.
PRECAUTIONS
• Handle pyridine with care.
• Owing to the presence of vapours of pyridine and butyl alcohol, it is preferable to carry out the tests in a fuming chamber.
• Old oils or oils having high diglyceride content give an abnormally high acetyl value.
