Table of Contents
INTRODUCTION
The saponification value serves as a crucial measurement, quantifying the quantity of potassium hydroxide (KOH) in milligrams necessary to completely saponify one gram of oil or fat. Saponification is the chemical reaction that occurs when fat undergoes treatment with an excess of alcoholic potassium hydroxide solution through refluxing. This process leads to the hydrolysis of triglycerides, resulting in the formation of glycerol and soap.
Determining the saponification value involves measuring the consumption of alkali during the hydrolysis process. This is achieved by titrating the excess alkali with standard hydrochloric acid, enabling the calculation of the saponification value.
By evaluating the saponification value, we gain insights into the chemical properties and composition of oils and fats. This measurement aids in understanding their suitability for various applications, such as soap manufacturing and culinary purposes. Additionally, it provides valuable information regarding the purity and quality of oils and fats, assisting in quality control processes within industries.
The saponification value serves as a fundamental parameter in the analysis of oils and fats, offering a quantitative measure of their chemical reactivity and potential applications.
The saponification value holds significant importance in the field of chemistry and industry, as it provides valuable insights into the characteristics and behavior of oils and fats. By determining the amount of potassium hydroxide required to saponify a given sample, we can assess its quality, purity, and suitability for various applications.
Saponification, the chemical process behind soap formation, involves the hydrolysis of triglycerides present in oils and fats. When treated with alcoholic potassium hydroxide solution under reflux conditions, the triglycerides break down into glycerol and soap molecules. The consumption of alkali during this hydrolysis reaction directly correlates with the saponification value.
Through meticulous titration of the excess alkali with standard hydrochloric acid, we can precisely measure the saponification value. This value serves as a reliable indicator of the average molecular weight of the fatty acids in the oil or fat sample. It allows us to understand the composition, structure, and overall quality of the lipid material under investigation.
The saponification value finds extensive applications in various industries. In the soap manufacturing industry, it helps determine the appropriate amount of alkali needed for the complete saponification of fats, ensuring the production of high-quality soap products. Moreover, the saponification value assists in the assessment of edible oils and fats, ensuring their suitability for culinary purposes and providing information about their nutritional content.
Additionally, the saponification value aids in quality control procedures, enabling comparisons between different oil and fat samples. It serves as a useful tool in identifying adulteration or the presence of impurities, ensuring the integrity and purity of the final products.
Overall, the saponification value plays a vital role in understanding the chemical nature of oils and fats, facilitating their effective utilization in a wide range of industrial, cosmetic, and culinary applications. Its determination serves as a key analytical technique for researchers, manufacturers, and quality assurance personnel in the field of lipid analysis.
PRINCIPLE
Apparatus
- Conical Flasks: Capacity of 250 to 300 ml.
- Reflux Condenser: Minimum length of 65 cm.
- Water-Bath or Electric Hot-Plate with Rheostat Control.
Reagents
- Alcoholic Potassium Hydroxide Solution: Dissolve 35 to 40 g of KOH in 20 ml of distilled water, then add sufficient aldehyde-free rectified spirit to make up to 1000 ml. Allow it to stand overnight, decant the clear liquid, and store it tightly closed in a bottle.
- Aldehyde-Free Rectified Spirit: Reflux 1.2 liters of rectified spirit for 30 minutes in a round-bottom flask with 10 g of KOH and 6 g of granulated aluminum (or aluminum foil). Distill and collect one liter after discarding the first 50 ml.
- Phenolphthalein Indicator Solution: Dissolve 1.0 g of phenolphthalein in 100 ml of rectified spirit. Note: For oils or fats producing dark-colored soap solutions, thymolphthalein or alkali blue 6B can be used instead of phenolphthalein. Additionally, 1 ml of a 0.1% (w/v) solution of methylene blue in water can be added to each 100 ml of phenolphthalein indicator solution before the titration.
- Standard Hydrochloric Acid (0.5 N).
PROCEDURE
- If the sample is not already in liquid form, melt it and filter it through a filter paper to remove any impurities and residual moisture. Ensure the sample is completely dry.
- Thoroughly mix the sample and accurately weigh approximately 1.5 to 2.0 g in a conical flask.
- Add 25 ml of the alcoholic KOH solution to the flask and connect the reflux air condenser.
- Heat the flask on a water-bath or electric hot plate for no more than 1 hour.
- Boil the mixture gently but steadily until the sample is fully saponified, indicated by the absence of any oily matter and the appearance of a clear solution.
- After the flask and condenser have cooled slightly, rinse the inside of the condenser with about 10 ml of hot ethyl alcohol neutral to phenolphthalein.
- Add approximately 1 ml of phenolphthalein indicator solution and titrate with standard hydrochloric acid.
- Perform a blank determination simultaneously with the sample.
CALCULATION

Where,
B = volume, in ml, of HCl required for the blank,
S = volume, in ml, of HCl required for the sample,
N = normality of HCl, and
W = weight, in g, of the material taken for the test.
RESULTS AND INFERENCE
Report the mean value of two determinations. The difference between two determinations carried out simultaneously or consecutively by the same analyst (repeatability).
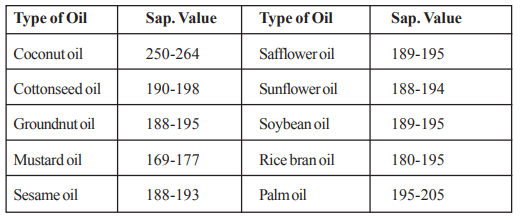
The saponification value of commonly used edible oils is given as below.
PRECAUTIONS
- Prepare the alcoholic KOH solution overnight.
- Refluxing should not exceed one hour.
- Thoroughly wash the condenser with alcohol to ensure cleanliness.
- Conduct the blank determination simultaneously with the sample for accurate results.
