Table of Contents
Introduction
Total Phenolic Content (TPC) refers to the concentration of phenolic compounds present in a given sample of food. These compounds are known for their antioxidant properties, which are essential for combating oxidative stress in the human body.
Phenolic compounds are natural antioxidants found in various foods, including fruits, vegetables, grains, and beverages. Their presence not only contributes to the sensory attributes of food but also plays a significant role in human health.
Determining TPC in food is crucial for several reasons:
- Assessing Nutritional Value: TPC analysis helps in evaluating the nutritional quality of food products. A higher TPC often indicates a richer source of antioxidants.
- Quality Control: Food manufacturers use TPC analysis to maintain consistent product quality and ensure that it meets regulatory standards.
- Health Benefits: Understanding TPC helps consumers make informed choices about their diets, as high TPC foods are associated with various health benefits.
- Research and Development: Scientists use TPC analysis to develop new food products with improved health attributes.
Significance of Phenolic Compounds in Food
The significance of phenolic compounds in food is that they can help to protect the body from damage caused by free radicals. Free radicals are unstable molecules that can damage cells and tissues, leading to various health problems. Phenolic compounds can scavenge free radicals and prevent them from causing damage.
In addition to their antioxidant properties, phenolic compounds have also been shown to have other health benefits, such as:
- Reducing inflammation
- Preventing cancer
- Boosting the immune system
- Protecting against heart disease
- Improving cognitive function
- Slowing down the aging process
The amount of phenolic compounds in food varies depending on the type of food. Some of the foods that are high in phenolic compounds include:
- Berries
- Nuts
- Seeds
- Vegetables
- Tea
- Coffee
- Cocoa
Increasing your intake of foods high in phenolic compounds can help improve your overall health and well-being.
Here are some specific examples of the health benefits of phenolic compounds:
- Antioxidant activity: Phenolic compounds can help to protect the body from damage caused by free radicals. Free radicals are unstable molecules that can damage cells and tissues, leading to a variety of health problems, including cancer, heart disease, and neurodegenerative diseases.
- Anti-inflammatory activity: Phenolic compounds can help to reduce inflammation. Inflammation is a natural response to injury or infection, but chronic inflammation can damage cells and tissues and lead to a variety of health problems.
- Anticancer activity: Phenolic compounds have been shown to have anticancer properties. They can help to prevent the formation of cancer cells, promote the death of cancer cells, and stop the spread of cancer cells.
- Immunomodulatory activity: Phenolic compounds can help to modulate the immune system. This means that they can help to boost the immune system or suppress it, depending on the need.
- Cardioprotective activity: Phenolic compounds can help to protect the heart and blood vessels. They can help to reduce the risk of heart attack, stroke, and other cardiovascular diseases.
- Neuroprotective activity: Phenolic compounds can help to protect the brain and nervous system. They can help to reduce the risk of Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases.
- Anti-aging activity: Phenolic compounds can help to slow down the aging process. They can help to protect cells from damage and promote cell regeneration.
Methods for Determination of Total Phenolic Compounds
When it comes to analyzing TPC in food, several methods are commonly employed. Each method has its advantages and limitations, making it essential to choose the most suitable one for the specific sample and research objectives. Here are some of the widely used methods:
Folin-Ciocalteu Method
The Folin-Ciocalteu method is a classic and widely adopted technique for TPC determination. It relies on the reaction between phenolic compounds and a reagent called Folin-Ciocalteu’s phenol reagent. The reaction produces a blue color, the intensity of which is directly proportional to the TPC.
High-Performance Liquid Chromatography (HPLC)
HPLC is a sophisticated analytical technique that offers high precision and accuracy in TPC determination. It involves the separation of phenolic compounds in a sample using a liquid chromatograph.
Spectrophotometry
Spectrophotometry is another commonly used method for TPC determination. It measures the absorption of light by phenolic compounds in the sample. The higher the absorption, the greater the TPC.
Colorimetric Assays
Colorimetric assays involve using specific color reactions to determine TPC. These assays are relatively simple and provide quick results.
Electrochemical Methods
Electrochemical methods, such as cyclic voltammetry and amperometry, are used to measure the electrochemical behavior of phenolic compounds. These methods are suitable for analyzing complex matrices.
Total Phenolic Content (TPC) in Food by Folin-Ciocalteu Method
Principle
The Folin-Ciocalteu method operates on the principle of quantifying the Total Phenolic Content (TPC) in a substance, particularly in food samples. It relies on a chemical reaction between phenolic compounds in the sample and the Folin-Ciocalteu reagent. This reaction results in the formation of a blue-colored complex. The intensity of this blue color directly correlates with the concentration of phenolic compounds in the sample.
In essence, the Folin-Ciocalteu method measures the strength of the color produced, and this color strength is a reflection of the phenolic content. By comparing this color intensity with a standard curve generated using known concentrations of a reference phenolic compound, one can precisely determine the TPC in the sample.
Chemical reactions involved
The chemical reaction underlying the Folin-Ciocalteu method involves the oxidation of phenolic compounds in the presence of a reagent composed of phosphotungstic and phosphomolybdic acids. This oxidation process results in the formation of a blue-colored complex.
The exact chemical reactions occurring during this process can be quite complex and are influenced by the specific phenolic compounds present in the sample. However, in simplified terms, it can be summarized as follows:
- Phenolic compounds in the sample (such as phenols and polyphenols) react with the Folin-Ciocalteu reagent.
- This reaction causes the reduction of the phosphomolybdic and phosphotungstic acids in the reagent.
- The reduced reagent then undergoes further chemical changes, leading to the formation of the blue complex.
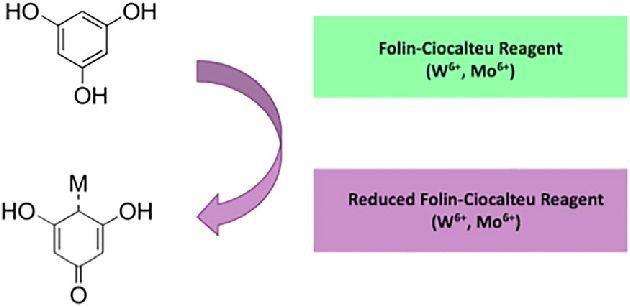
The intensity of this blue color is directly proportional to the concentration of phenolic compounds in the sample. Therefore, by measuring the absorbance of this blue complex at a specific wavelength, typically around 760 nm, scientists can quantify the total phenolic content in the sample.
Detailed Procedure
The procedure for determining Total Phenolic Content (TPC) using the Folin-Ciocalteu (FC) method involves several steps:
Materials and Reagents:
- Sample of interest (e.g., food extract)
- Folin-Ciocalteu reagent
- Sodium carbonate (Na2CO3) solution
- Distilled water
- Test tubes or cuvettes
- Spectrophotometer
- Pipettes and pipette tips
- Glassware (flasks, beakers)
- Vortex mixer or shaker
- Protective gear (lab coat, gloves, safety goggles)
Procedure:
- Sample Preparation:
- Weigh an accurately measured amount of your food sample. Typically, you’ll use a food extract or solution. Ensure that the sample is properly homogenized. If your sample extract is not prepared, then prepare the extract first by below procedure:
- Take 0.6g of your sample and 15ml of 80% methanolic solution in a centrifuge tube ( 80% methanolic solution means add 12ml of methanol in 3 ml of distilled water). If you have more samples, you can increase the quantity accoringly.
- Now mix in a vortex mixer and incubate for 1hour at 50 degree C in an incubator.
- Centrifuge at 3000g for 10minutes and then collect the supernatent.
- You will use this supernatent now
- Weigh an accurately measured amount of your food sample. Typically, you’ll use a food extract or solution. Ensure that the sample is properly homogenized. If your sample extract is not prepared, then prepare the extract first by below procedure:
- Prepare Standard Solutions:
- Prepare a series of standard solutions of a known phenolic compound (e.g., gallic acid) with varying concentrations. These standards will be used to create a calibration curve.
- Example
- How to prepare the standard- See the complete step by step guide to How to prepare Gallic acid standard for Total phenolic content (TPC) estimation in food
- Suppose you are examining coffee beans and you have treated ground coffee samples for 0hr, 2 hrs, 4hrs, 8hrs and 10hrs with Ultrasonic waves to see the effect of it. So, these are our different samples
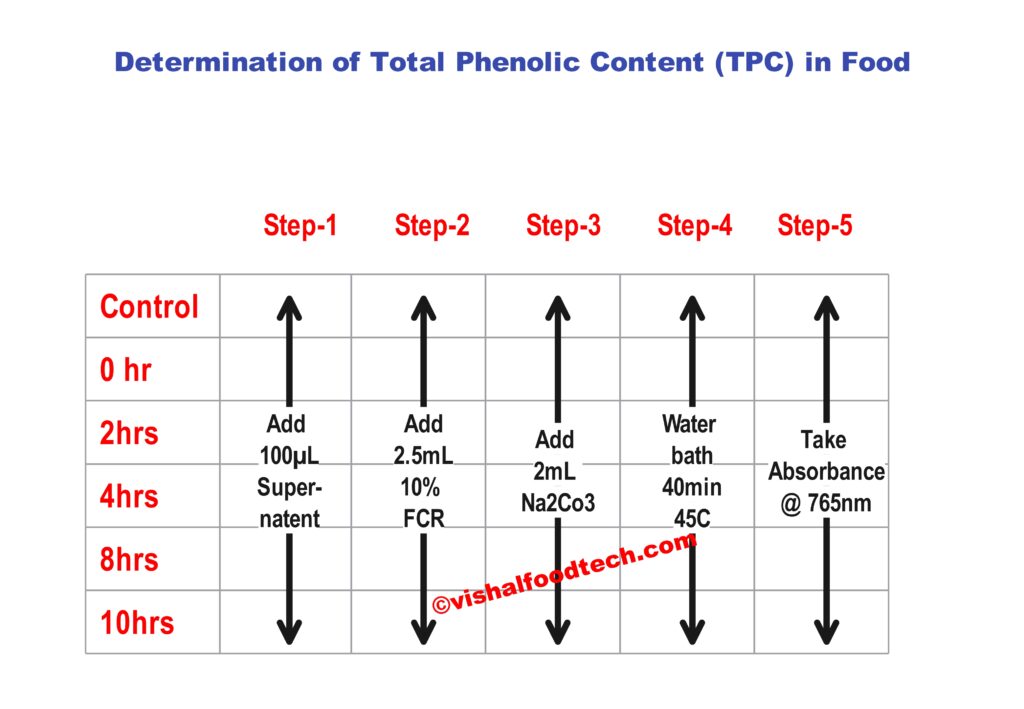
- Reaction with Folin-Ciocalteu Reagent:
- In separate test tubes, place your sample and the standards.
- To each test tube, add an appropriate volume of the Folin-Ciocalteu reagent.
- Incubation:
- Allow the test tubes to stand for a specific period, typically around 5-10 minutes, at room temperature. During this time, a color change should occur, indicating the formation of the blue complex.
- Add Sodium Carbonate Solution:
- After the incubation period, add a measured volume of sodium carbonate (Na2CO3) solution to each test tube. This solution acts as a stabilizer and pH adjuster.
- Mix Thoroughly:
- Ensure that the contents of each test tube are well-mixed, either by vortexing or using a shaker.
- Incubation (Again):
- Allow the test tubes to incubate in the dark for an additional period, typically 30-60 minutes, at room temperature. This extended incubation allows the color to develop fully.
- In The Standards also, you have to add all the reagents as shown below in all steps
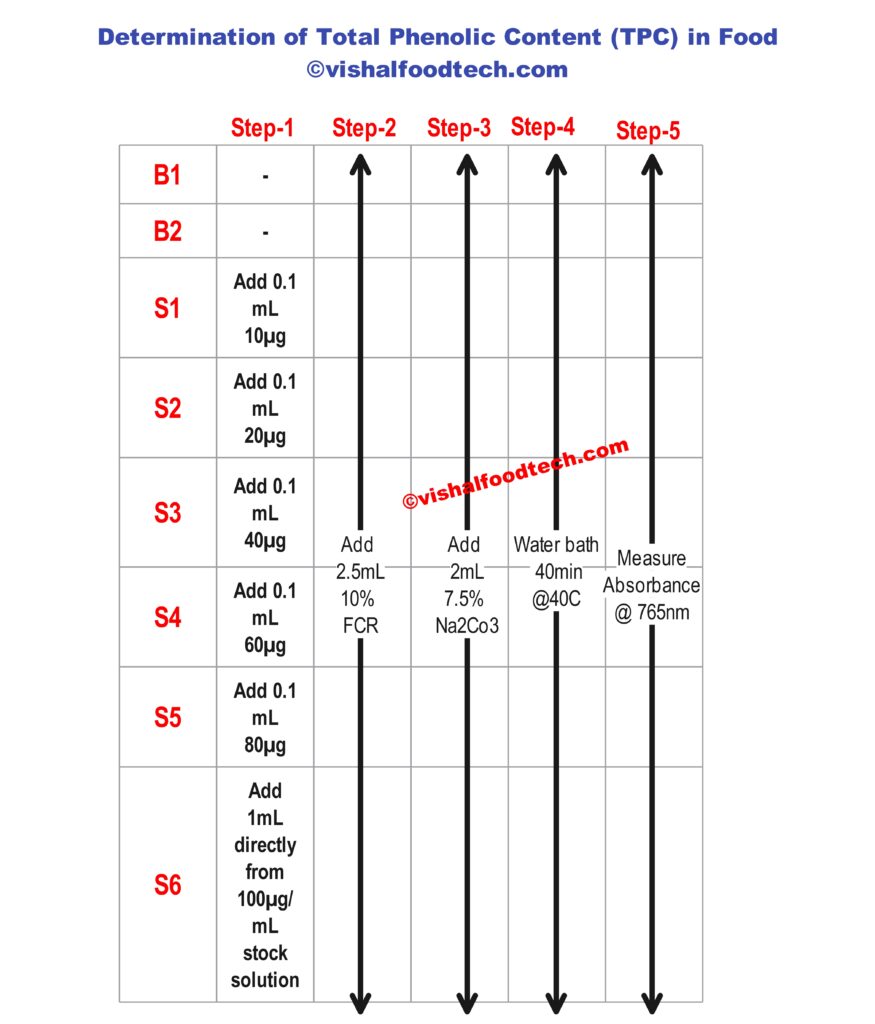
- Measure Absorbance:
- Use a spectrophotometer to measure the absorbance of the blue complex at the appropriate wavelength, which is usually around 760 nm.
- Calibration Curve:
- Create a calibration curve using the absorbance values of the standard solutions. Plot concentration against absorbance to establish a linear relationship.
- Determine TPC:
- Use the calibration curve to determine the TPC in your sample by comparing its absorbance value to the curve. The TPC is typically expressed in terms of the equivalent concentration of the phenolic compound used for the standards.
- Report Results:
- Express the TPC in your sample in appropriate units (e.g., milligrams of gallic acid equivalents per gram of sample, mg GAE/g).
- Clean Up:
- Properly dispose of any chemical waste and clean your equipment thoroughly.
Remember that the accuracy of your TPC determination relies on precise measurements and adherence to the procedure. It’s essential to handle chemicals safely and follow laboratory safety guidelines throughout the process.
