Carbohydrate analysis is a crucial aspect of studying and understanding the composition and structure of various substances. One method that stands out is the determination of Carbohydrate by Phenol-Sulfuric Acid Method. This method offers a reliable and widely used approach for quantifying carbohydrates present in a sample. By harnessing the power of chemical reactions, the phenol-sulfuric acid method enables researchers to accurately determine the carbohydrate content and explore their implications in various fields such as food science, biochemistry, and pharmaceutical research. In this blog, we will delve into the intricacies of carbohydrate analysis by exploring the phenol-sulfuric acid method, its principles, applications, and best practices.
Table of Contents
Do you know the Principle ?
The principle lies in the reaction between carbohydrates and concentrated sulfuric acid in the presence of phenol. This reaction results in the formation of furfural compounds from the carbohydrates, and these furfural compounds further react with phenol to create a colored complex.
Key steps:
- Initial Reaction: When concentrated sulfuric acid is added to a carbohydrate-containing sample, it dehydrates the carbohydrates, breaking them down into simpler compounds, primarily furfural and other intermediates. This step is critical as it converts carbohydrates into compounds that can react further.
- Formation of Furfural Compounds: Furfural compounds are produced as a result of the dehydration reaction. These compounds are key intermediates in the subsequent color-forming reaction with phenol.
- Reaction with Phenol: Phenol is added to the reaction mixture. Furfural compounds react with phenol to create a complex mixture of compounds, including colored products.
- Color Development: The complex mixture formed in the presence of phenol includes compounds that absorb light in the visible spectrum. This absorption results in the development of a color directly proportional to the concentration of carbohydrates in the sample.
- Spectrophotometric Analysis: The colored solution is then subjected to spectrophotometric analysis. A spectrophotometer measures the absorbance of light by the solution at a specific wavelength, usually between 480-490 nm. The intensity of this absorbance is directly related to the concentration of carbohydrates in the sample.
By comparing the absorbance of the sample to a standard curve created with known concentrations of a standard carbohydrate solution, the concentration of carbohydrates in the sample can be accurately determined.
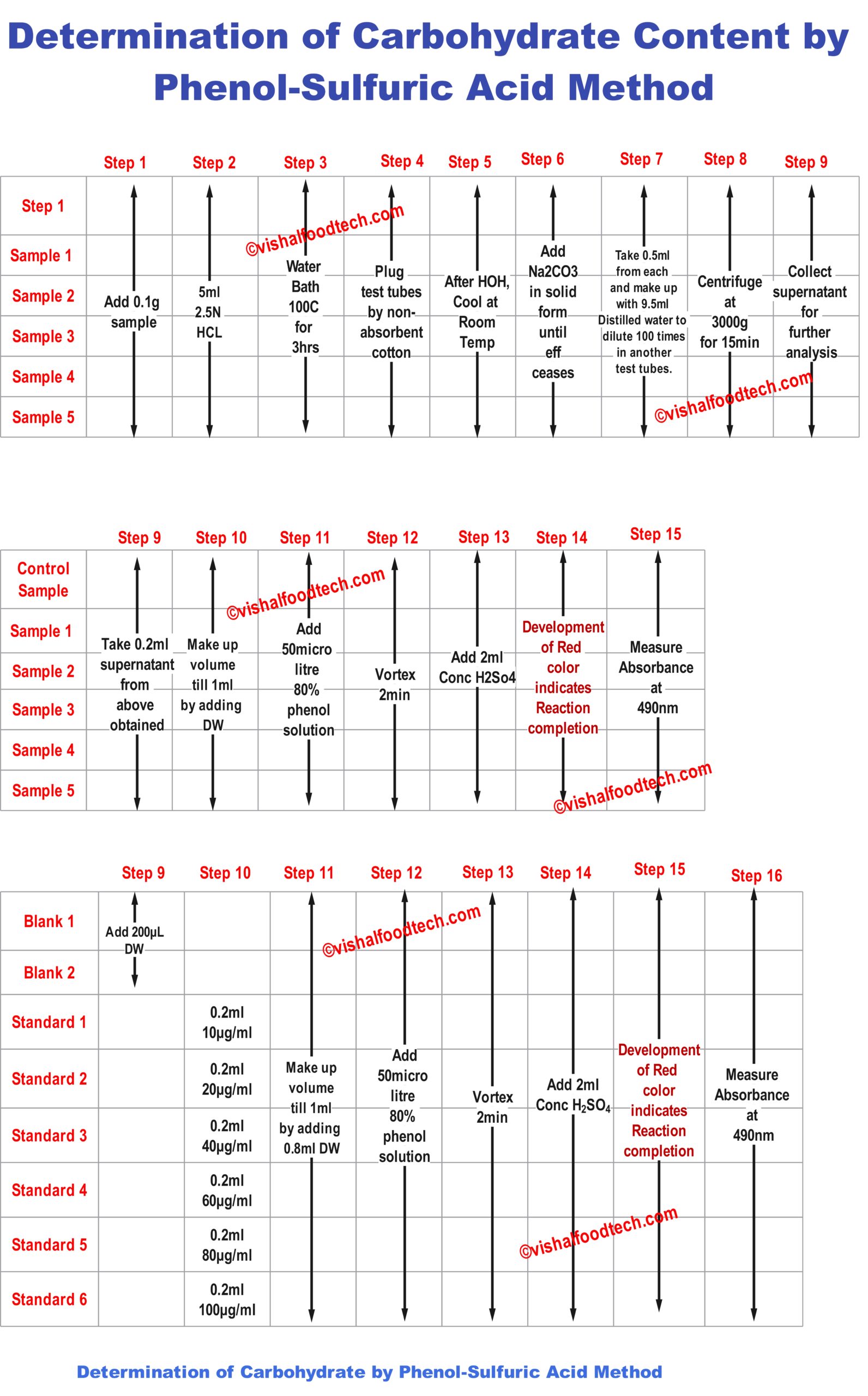
FOR GROUND POWDERED SAMPLE:
Sample preparation for Determination of Carbohydrate by Phenol-Sulfuric Acid Method

- 0.1 g of ground samples were taken in test tubes;

- The samples were hydrolyzed by using 5 ml of 2.5 N Hydrochloric acid (HCI) solution in the water bath at temperature of 100 °C ± 1 °C for 3 hrs.
Preparation of 2.5 N Hydrochloric acid (HCI) solution
Density= (Mass/ Volume)
We have to prepare 2.5 N Hydrochloric acid (HCI) solution.
We know Normality=(Acidity or basicity)* Molarity
HCL has an acidity of 1 because it can release only one proton (H+).
So from the above formula, for HCL, Normality= Molarity (bez Acidity =1)
Hence, 2.5N=2.5M

So, we can take the Volume of solution in litre=1 L and Molarity = 2.5M in the above formula, and no of moles comes out as 2.5 moles.
now Density= (Mass/ Volume)——-1

Putting all these values, density of HCL= 1.18, Mol wt of HCL= 36.458,
We can find Volume = (2.5*36.458)/1.18 = 77.24ml per 1 Litre H2O.
Since HCL was of 35% purity, divide by 0.35 >> (77.24/0.35)= 220ml/ 1000mL H2O. ( So, to make 2.5N HCL, take 220mL in 1000mL DW- distilled water, or if we want to make a smaller amount, we can take 22mL in 100mL DW, or even 2.2mL in 10mL DW)
So now added 5mL of that prepared 2.5N HCL solution in the test tubes. (See figure below)

- All these centrifuge tubes were transferred to testtubes and were kept in the water bath at 100 degrees Celsius for 3 hours.


- The test tubes were plugged by using non-adsorbent cotton. After hydrolysis, the samples were allowed to cool down at ambient room temperature.
After 3 hours, color changed to deep iron red as shown below in figure:

- Sodium carbonate in solid form was added to the test tubes, until the effervescence ceases. Finally, the volume was made up to 100 mL by adding distilled water.


- Now take 0.5ml from each and make up with 9.5ml Distilled water to dilute 100 times in another test tubes.
- The samples were centrifuged at 3000×g for 15 minutes. The supernatant was collected for the carbohydrate estimation.
- Collect the supernatant for the carbohydrate estimation. This supernatant will be further used.
- Below is the detailed step-by-step procedure till Supernatant collection.
Standard Curve Generation for the Determination of Carbohydrate by Phenol-Sulfuric Acid Method

Now the below-shown figure is of samples after the Concentrated sulphuric acid addition.

You can now plot the standard curve and get the value of x which is concentration of carbohydrates present in your sample.

