Table of Contents
What are the Standards in Spectrophotometry?
In spectrophotometry, a “Gallic acid standard” or any general standard typically refers to a sample of known concentration or properties used as a reference for comparison when measuring the concentration or properties of an unknown sample. These standards are crucial for calibrating the spectrophotometer and for quantifying the concentration of substances in the sample being analyzed.
Here’s how standards are used in spectrophotometry:
- Calibration: Spectrophotometers measure the absorption or transmission of light by a sample at specific wavelengths. To ensure accurate measurements, the spectrophotometer needs to be calibrated using standards of known concentrations. These standards are used to create a calibration curve or standard curve, which relates the absorbance or transmittance of light to the concentration of the substance being analyzed.
- Quantification: Once the spectrophotometer is calibrated, it can be used to determine the concentration of the substance in an unknown sample. By measuring the absorbance or transmittance of light for the unknown sample and then using the calibration curve, you can calculate the concentration of the substance in the sample.
- Quality Control: Standards are also used for quality control purposes. By regularly measuring the absorbance or transmittance of standard solutions, laboratories can monitor the performance and accuracy of their spectrophotometers over time. Any deviations from expected results may indicate a need for maintenance or recalibration.
- Method Validation: When developing a new analytical method in spectrophotometry, standards are used to validate the method’s accuracy and reliability. Researchers analyze standard samples to ensure that the method produces consistent and accurate results for known concentrations before applying it to unknown samples.
Why Gallic Acid is Used as a Standard in Total Phenolic Content (TPC) Determination?
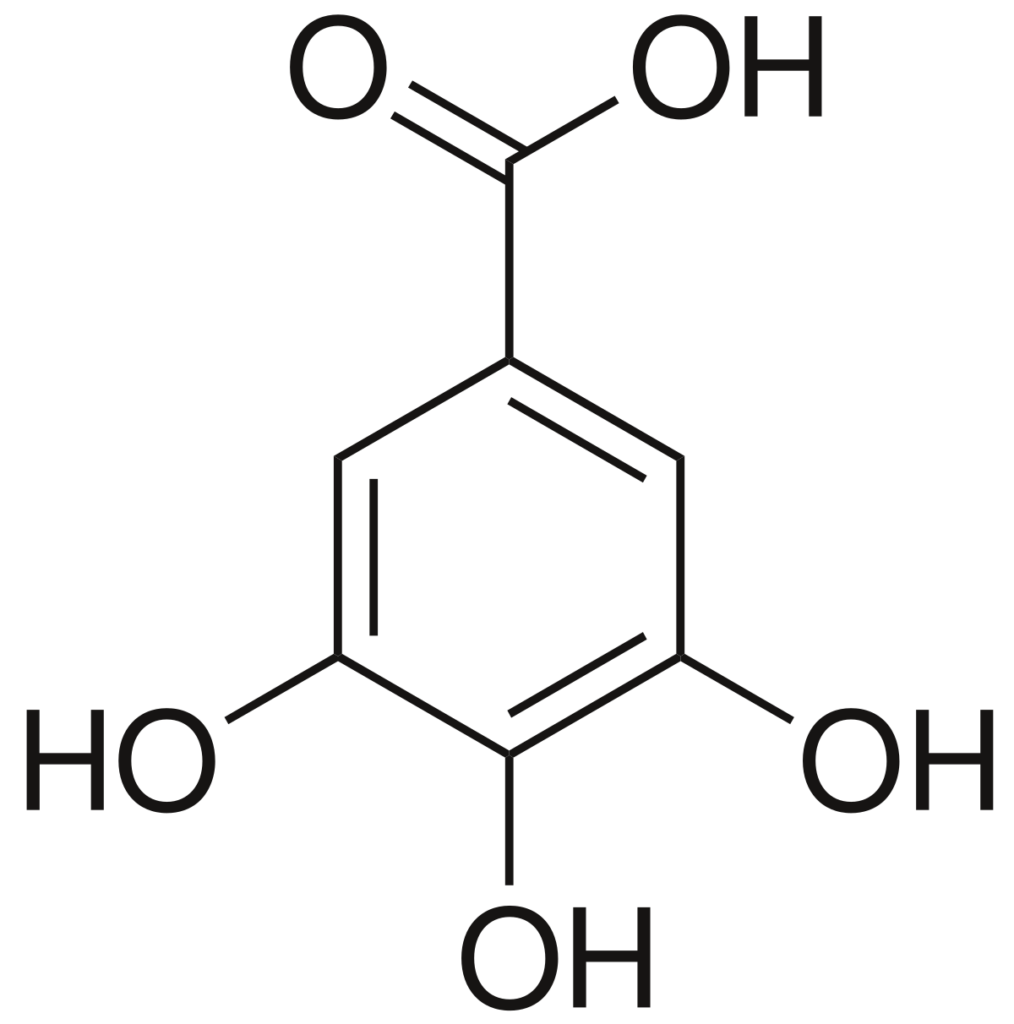
Gallic acid is commonly used as a standard in the determination of Total Phenolic Content (TPC) for several reasons:
- Stability: Gallic acid is relatively stable under various experimental conditions, making it a suitable choice for a standard. It doesn’t easily undergo chemical reactions or degradation during the analysis process, ensuring results’ accuracy and consistency.
- Solubility: Gallic acid is highly soluble in common solvents like water, ethanol, and methanol. This solubility makes preparing standard solutions of known concentrations easy, simplifying the calibration process for spectrophotometers or other analytical instruments.
- Availability: Gallic acid is readily available and relatively inexpensive, making it accessible for research and analytical purposes. Researchers can easily obtain pure gallic acid for use as a standard.
- Spectral Characteristics: Gallic acid has well-defined spectral characteristics in the UV-Vis range, which is commonly used in spectrophotometric analysis. Its absorbance properties at specific wavelengths make it suitable for creating calibration curves and accurately quantifying phenolic compounds in samples.
- Similarity to Phenolic Compounds: Gallic acid shares structural similarities with many phenolic compounds found in natural sources, such as fruits, vegetables, and plant extracts. Using gallic acid as a standard allows for a reasonable approximation of the phenolic content in these complex samples.
- Reproducibility: Gallic acid standards have been widely used in the scientific community for TPC determination. This widespread use contributes to the reproducibility of results across different laboratories and studies.
- Well-characterised: Gallic acid has well-established chemical properties and behaviour, making it easier to validate analytical methods and ensure accurate TPC measurements.
Detailed Procedure-
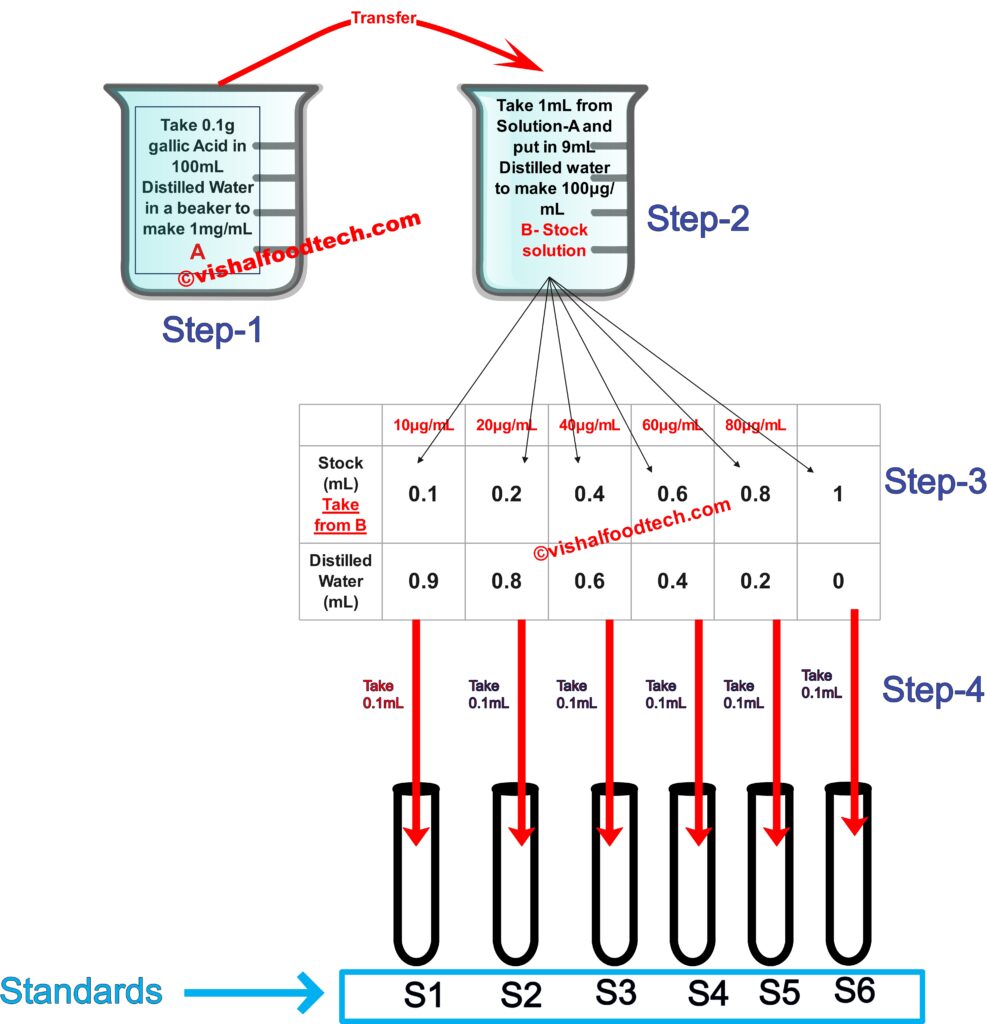
Step-1
0.1g is dissolved in 100ml Distilled water, so dividing 0.1 by 100, we get the concentration as 0.001g/mL or we can write as 1mg/mL
Step-2
Now, transfer 1mL in another beaker B containing 9mL distilled water. Since, 1mL+ 9mL make total volume to 10mL, that 1mg/mL added had 1mg in 1ml of solution A according to the concentration of 1mg/mL so that it will remain the same in the 10mL solution now. So, we can say that 1mg is dissolved in 10mL of solution hence, dividing 1mg by 10mL gives 0.1mg/mL or can be written as 100µg/mL, as of B-stock solution shown in Step-2.
Step-3
Take 0.1mL from B-stock solution of 100µg/mL and make up the volume to 1mL by adding 0.9mL Distilled water. 100µg/mL solution means in 1mL, 100µg of solute is present, but I have pipetted out 0.1mL solute from it, so in 1mL, 100*0.1= 10µg/mL concentration is present.
Similarly, in Standards S2, 0.2mL of 100µg/mL solution is added and the final concentration of the resulting standard S2 is 20µg/mL.
Similarly, we get 40,60 and 80µg/mL for S3, S4 AND S5 respectively.
Now, gallic acid standard preparation is complete, If you are using this standard for TPC i.e. Total phenolic content, further add FCR and Na2Co3 and then keep in water bath for 45 minutes at 45 degree Celsius for proper development of the blue color and then read the absorbence at 765nm wavelength to finally plot a graph between Absorbance and concentration.
