Table of Contents
INTRODUCTION
In the field of chemistry, the iodine value is a crucial parameter used to determine the degree of unsaturation in glycerides of unsaturated fatty acids. It provides valuable insights into the composition and properties of various oils and fats.
By measuring the iodine value, we can gain a deeper understanding of the chemical structure and potential applications of these substances in foods.

PRINCIPLE
The iodine value test relies on the reaction between the unsaturated fatty acids and iodine monochloride solution (known as Wijs solution) in a carbon tetrachloride medium.
The iodine monochloride reacts with the double bonds present in the unsaturated fatty acids, forming diiodo compounds. The excess iodine monochloride is then treated with potassium iodide, liberating iodine, which can be quantitatively determined through titration with sodium thiosulphate solution.
REQUIREMENTS
Reagents
To perform the iodine value test, several reagents are required:
- Potassium Dichromate
- Concentrated Hydrochloric Acid
- Potassium Iodide Solution: Prepare a fresh solution by dissolving 10 g of KI, ensuring it is free from potassium iodate, in 90 ml of water.
- Starch Solution: To prepare, triturate 5 g of starch and 0.01 g of mercuric iodide with 30 ml of cold water. Slowly pour this mixture into one liter of boiling water, boil for three minutes, allow it to cool, and decant the clear liquid.
- Standard Sodium Thiosulphate Solution (0.1N)
- Glacial Acetic Acid
- Iodine Monochloride (ICl) – 98%
- Wijs Iodine Monochloride Solution: Dissolve 10 ml of iodine monochloride in approximately 1800 ml of glacial acetic acid and shake vigorously. Pipette 5 ml of this solution, add 10 ml of KI solution, and titrate with 0.1 N standard Na2S2O3 solution, using starch solution as an indicator. Adjust the volume of the solution until it is approximately 0.2N.
- Carbon Tetrachloride or Chloroform: Inert solvents compatible with Wijs solution.
PROCEDURE
- Ensure the sample is completely liquid, and filter it through a filter paper to eliminate impurities and any residual moisture.
- Thoroughly clean and dry both the sample and the glass apparatus used for the test.
- Weigh a precise quantity of the oil or fat sample, which will be dissolved in a clean, dry 500 ml iodine flask or a well-ground glass-stoppered bottle containing 25 ml of carbon tetrachloride.
- Agitate the mixture to dissolve the contents, and then add 25 ml of Wijs solution. Wet the glass stopper with KI solution and replace it securely.
- Swirl the mixture for proper mixing, and allow it to stand in the dark for 30 minutes for non-drying and semi-drying oils, or 1 hour for drying oils.
- Simultaneously, perform a blank test under similar experimental conditions.
- After the designated time, add 15 ml of KI solution and 100 ml of water, ensuring the stopper is rinsed as well. Titrate the liberated iodine with standard Na2S2O3 solution, continuously swirling the bottle to avoid local excess, until the solution turns straw yellow and the iodine color is no longer visible.
- Add 1 ml of the starch solution as an indicator and continue the titration until the blue color formed disappears completely after thorough shaking with the stopper on.
CALCULATION
To calculate the iodine value, the following formula is used:
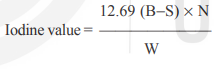
Where,
- B represents the volume, in milliliters, of Na2S2O3 solution required for the blank test.
- S represents the volume, in milliliters, of Na2S2O3 solution required for the sample.
- N represents the normality of the Na2S2O3 solution.
- W represents the weight, in grams, of the material taken for the test.
RESULTS AND INFERENCE
It is recommended to report the mean of the results obtained from two determinations. The difference between these two determinations, carried out simultaneously or in rapid succession by the same analyst (repeatability), should not exceed 0.5.
The iodine value ranges differ for various substances:
- Animal fats typically have an iodine value between 30 and 70.
- Non-drying oils have iodine values ranging from 80 to 110.
- Semi-drying oils exhibit iodine values ranging from 80 to 140.
- Drying oils have higher iodine values, ranging from 125 to 200.
- Waxes generally possess very small iodine values.
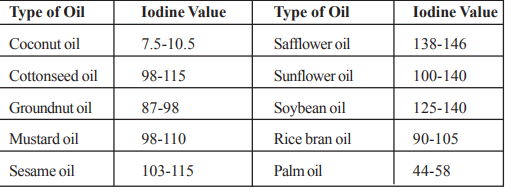
The iodine value of commonly used edible oils is given below.
Here are some examples of iodine values for commonly used edible oils:
- Olive Oil: Iodine Value – 80-88
- Coconut Oil: Iodine Value – 7-10
- Sunflower Oil: Iodine Value – 125-150
- Soybean Oil: Iodine Value – 120-143
- Canola Oil: Iodine Value – 94-130
- Corn Oil: Iodine Value – 120-140
- Peanut Oil: Iodine Value – 80-110
- Sesame Oil: Iodine Value – 103-120
PRECAUTIONS
During the iodine value test, it is essential to take certain precautions to ensure accurate and reliable results:
- As soon as the Wijs solution is added, immediately stopper the flask to prevent the escape of iodine vapor.
- Maintain the flask in a dark environment for precisely 30 minutes or the designated time specified for the particular type of oil or fat being tested.
- Ensure precise and accurate weighing of the sample based on the expected iodine value.
- If the difference (B-S) between the blank and sample titration volumes is greater than half of the blank volume (B/2), repeat the test using a smaller quantity of the sample.
Conclusion
The iodine value test is a valuable analytical method for determining the degree of unsaturation in oils and fats. By measuring the amount of iodine that reacts with unsaturated fatty acids, we can gain insights into their composition and characteristics.
Through careful procedure, calculation, and analysis, the iodine value provides meaningful information for various applications in the food, cosmetic, and pharmaceutical industries. Understanding the iodine value allows us to make informed choices regarding the suitability and stability of different oils and fats in different formulations and processes.
