Table of Contents
INTRODUCTION
Besides extending the shelf life of a food, a chemical preservative should be safe for human consumption, should not impart undesirable organoleptic changes, be economical in use and be capable of being analysed.
While the use of chemical preservative to be safe under conditions of use is governed by law, it is considered necessary to prescribe methods for their analysis. Sorbic acid, a chemical preservative should not be used in food.
PRINCIPLE
Sorbic acid or its salt is extracted from food products by steam distillation and then to the distillate, acidified potassium dichromate and thiobarbituric acid are added.
The developed color in solution is read in a spectrophotometer at 532 nm against blank.
REQUIREMENTS
Apparatus
- Steam distillation apparatus
- Volumetric flasks (50 ml and 1 litre capacity)
- Volume pipettes (5 ml and 20 ml capacity)
- Test tubes (15 ml capacity)
- Spectrophotometer
- Water bath
Reagents
Sulphuric acid : 2N and 0.3N.
Potassium dichromate solution- 147 mg K2 Cr2 O7 dissolved in distilled water and diluted to 100 ml.
Thiobarbituric acid solution 0.5% – Dissolve 250 mg thiobarbituric acid in 0.5N NaOH solution in a 50 ml volumetric flask by swirling in hot water. Add 20 ml distilled water, neutralize with 3 ml of 1N HCl and dilute to volume with distilled water. This solution should be prepared fresh.
Crystalline magnesium sulphate MgSO4 .7H2 O.
Standard sorbic acid solution – Accurately weigh 134 mg potassium sorbate (equivalent to 100 mg sorbic acid) and dilute to 1 litre with distilled water. One ml of solution corresponds to 0.1 mg of sorbic acid. This solution is stable for several days when refrigerated.
PROCEDURE
- Weigh 1.5 to 2.0 g prepared sample into distillation tube containing silicon chips.
- Add 10 ml of 2N H2 SO4 solution and 10 g magnesium sulfate.
- Steam distill the contents, maintaining 20-30 ml volume in distillation tube with small burner.
- Avoid charring. Collect 100-125 ml distillate in 250 ml volumetric flask within 45 min.
- Rinse the condenser with distilled water and dilute the distillate to volume and mix thoroughly.
- Pipette 2 ml of test portion and 2 ml of distilled water (for blank) into separate 15 ml test tubes.
- Add 1 ml of 0.3N sulphuric acid and 1 ml of potassium dichromate solution and heat in a boiling water bath exactly for 5 min. Immerse tubes in an ice bath and add 2 ml thiobarbituric acid solution.
- Replace it in a boiling water bath and boil it for 10 min.
- Cool and determine the absorbance of the solution at 532 nm against blank using 1-cm cells.
- For the calibration curve, pipette 5, 10, 15, 20, and 25 ml of sorbic acid standard solutions into separate 500-ml volumetric flasks.
- Dilute each to volume and mix thoroughly and proceed as the sample.
- Plot the absorbance against μg sorbic acid/ml.
- Calculate the sorbic acid content in the sample after reading the corresponding sorbic acid value of the absorbance at 532 nm.
CALCULATION
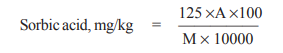
Where,
A = concentration of sorbic acid obtained from calibration curve, and
M = mass, in g, of sample taken.
RESULTS AND INFERENCE
The difference between the results of two determinations of sorbic acid carried out simultaneously or in rapid succession by the same analyst shall not exceed 0.01% by mass.
The use of sorbic acid in some food products is permitted by PFA Act but the concentration of sorbic acid is restricted to a specific level.
- Cheese or processed cheese – 3000 ppm (max.)
- Flour confectionery – 1500 ppm (max.)
- Preserved chapatis – 1500 ppm (max.)
- Paneer and chhana – 2000 ppm (max.)
- Fat spread – 1000 ppm (max.)
- Jam, jellies, candied peels, fruit bars – 500 ppm (max.)
- Fruit juice concentrate – 100 ppm (max.)
- Fruit juices – 200 ppm (max.)
- Nectars, ready-to-serve beverages – 50 ppm (max.)
PRECAUTIONS
• Thoroughly washed glassware should be used.
• Prepare standard solutions from a standard of known purity accurately
